Tips for Interpreting GC-MS Fragmentation of Unknown Substituted Fentanyls
Tips for Interpreting GC-MS Fragmentation of Unknown Substituted Fentanyls
2017-08-23
Holly Grenke Pierzynski1, M.S., Laura Neubauer1, Chung Choi1, Roxanne Franckowski1, M.S., Steven D. Augustin2, M.S., and Donna M. Iula1, Ph.D.
1Cayman Chemical and 2 Velesco Pharmaceutical Services
Fentanyl is a powerful synthetic opioid developed by Janssen Pharmaceutica in 1959 that has roughly one hundred times the potency of morphine. Due to fentanyl’s powerful analgesic properties and wide therapeutic index, it has been safely used by the medical community for decades. Structurally, fentanyl can be described as a 4-anilidopiperidine. Extensive structure-activity relationship (SAR) studies are well documented in scientific and patent literature.1-3 Some additional 4-anilidopiperidines are safely used in human and/or veterinary medicine (such as alfentanil, sufentanil, and remifentanil) but most have never advanced to clinical trials.4,5 Until recently, only a handful of 4-anilidopiperidines, such as 3-methyl fentanyl, acetyl fentanyl, and β-hydroxythiofentanyl, were found on the illicit market for recreational drug use within the U.S. and overseas.6,7 Beginning in late 2015 to early 2016, numerous additional fentanyl-like compounds began to emerge, and there has been no hint of this trend slowing down. The rapid proliferation on the gray market of new molecular entities containing the fentanyl scaffold—designed to skirt existing regulations—has certainly made the identification of new psychoactive substances a challenge (Figure 1). The majority of the new fentanyl varieties have been modified at the regions defined on the fentanyl scaffold below.
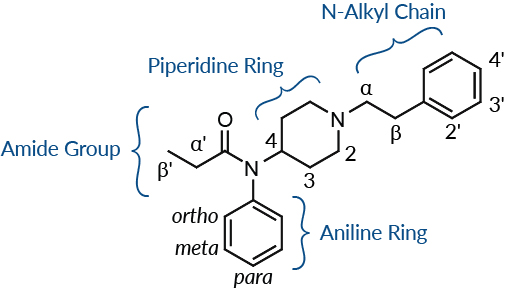
Figure 1. Cayman's standardized naming convention differentiates the points of substitution on the fentanyl scaffold by region.
Typical variation swaps are noted in the table below.
| Region | Typical Variations |
|---|---|
| Amide Group |
|
| Aniline Ring |
|
| Piperidine Ring |
|
| N-Alkyl Chain |
|
The obvious challenge for law enforcement and border patrol is the ability to identify new unknown substances quickly and accurately. To do so, they often turn to GC-MS libraries for a match. However, if the compound is so novel that mass spectra data has not yet been collected, forensic scientists are left to rely on their chemistry knowledge to decipher clues as to what functional groups have been substituted onto the fentanyl scaffold. Cayman Chemical’s scientists work hard to be at the forefront of identifying emerging drugs of abuse by quickly making authentic reference standards available. As new forensic materials go through the quality control process, the GC-MS data are added to the Cayman Spectral Library. By studying the GC-MS spectra of more than 50 fentanyl-like compounds, four major predictive patterns have emerged that provide clues to help in identifying unknown cases.
Pattern 1:
Fentanyl-like compounds cleave between the α and β carbons of the ethyl heterocyclic linker, which results in the base peak (BP) ion.
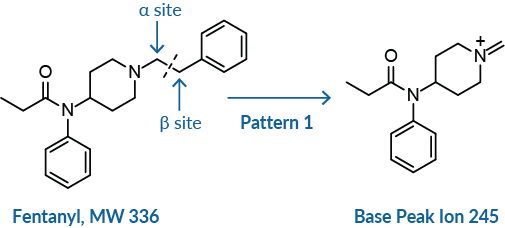
Often with fentanyl compounds, the molecular ion is not detected in the spectra, so the molecular weight (MW) of the compound is not easily discernable. In such cases, the base peak (BP) corresponds to the primary cleavage of the compound, which occurs between the α/β site on the phenethyl moiety or similar chain.
The presence of 91, the tropylium ion, in the spectra of fentanyl-like compounds strongly suggests a phenethyl group. In such cases, the MW of the compound will be equal to the BP plus 91. In the case of fentanyl, the molecular ion (336) is not discernable, but the BP is 245, so 245 + 91 = 336 (BP + CH2 Ph = MW) (Figure 2).8,9 When a linker other than a phenethyl is attached to the piperidine ring such as furanylethyl fentanyl, for example, the ion fragment for 91 is not observed. In this case, an ion fragment at 81 is observed instead for the furanylmethyl ion.
For substituted fentanyls with a hydroxyl group in the β position, such as β-hydroxy fentanyl and β-hydroxythiofentanyl, primary cleavage still occurs between the α/β site, but a smaller fragment peak will be observed as the MW minus 18 (M-18) for the loss of water. For β-hydroxy fentanyl that fragment is 334, and for β-hydroxythiofentanyl that fragment is 340. These peaks have the potential to be confused with the molecular ion, but according to Pattern 1, the exact molecular ion is not usually observed.
A noteworthy exception to this first pattern occurs when the phenethyl moiety is replaced with a N-benzyl or N-methyl moiety, as with benzyl fentanyl.8 In the case of benzyl fentanyl, the molecular ion (322) is noticeable along with the BP of 91, resulting from the cleavage between the piperidine ring and the benzyl group (Figure 2). In variations where the compound has a thienyl group instead of a benzyl, such as thienyl fentanyl, the BP is observed at 97.
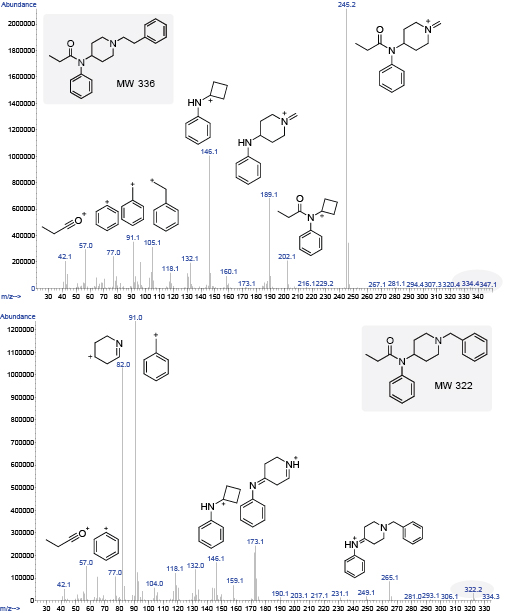
Figure 2. A comparison of the GC-MS of fentanyl with its corresponding ion fragments (left) and the GC-MS of benzyl fentanyl (right).
Pattern 2:
Additional cleavage of the BP ion occurs along the piperidine ring and at the amide C-N bond.
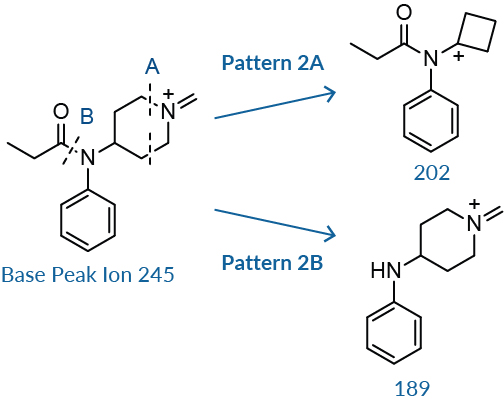
The BP ion undergoes additional cleavage in two areas. The first is along the piperidine ring, Pattern 2A, eliminating the nitrogen and two carbons to form a cyclobutyl group (mass 202 for fentanyl).8,10 This is a minor peak in abundance compared to the peak at 189 and 146 for fentanyl. The second cleavage occurs along the C-N amide bond to produce a peak with an abundance of 30-60% of the BP (mass 189 for fentanyl), Pattern 2B. 8 These masses will change depending on the substitutions on the piperidine ring, amide group, or aniline ring. Subtracting the mass of this fragment from the BP will yield the mass of the acylium ion.
Pattern 3:
Subsequent cleavage at either the piperidine ring or the amide C-N bond of the secondary fragments results in a third characteristic fragment.
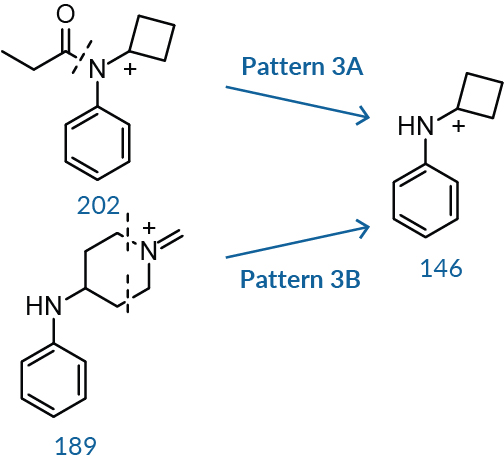
The third characteristic fragment results from cleavage along the amide bond and additional cleavage within the piperidine ring. In the case of fentanyl, cleavage of the amide C-N bond of ion 202, Pattern 3A, yields ion fragment 146. Similarly, cleaving the piperidine ring of ion 189, Pattern 3B, eliminates the nitrogen and two adjacent carbons, causing the ring to collapse into a cyclobutyl group and yielding the same ion 146.8,10 The ratio between 146 and 189 is almost always greater than one unless there is a branched alkyl group or a closed alkyl ring system, as seen with isobutyryl fentanyl and cyclopentyl fentanyl (Figure 3).
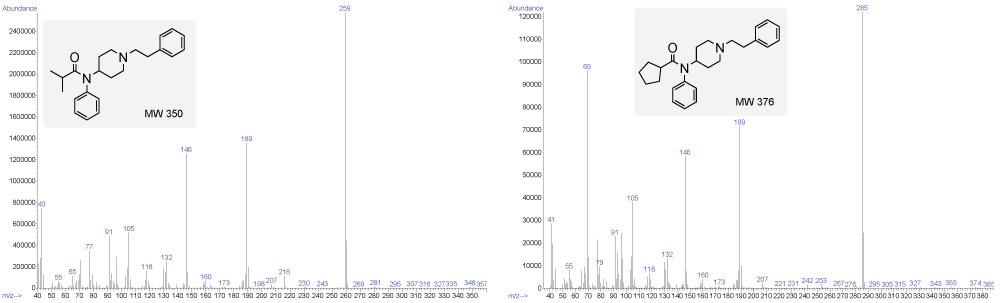
Figure 3. The GC-MS for isobutyryl fentanyl (left) is showing a 1:1 ratio of ions 146 to 189, while the GC-MS for cyclopentyl fentanyl (right) is showing a ratio of less than one, where the ion 189 is in larger abundance than the ion 146.
Thus, if the 146 and 189 peaks are present in the GC-MS spectra, your unknown will most likely be a fentanyl-like compound without substitutions on the piperidine or aniline regions. If the mass fragments 146 and 189 are absent, this will likely indicate a substitution on the aniline or piperidine ring. Using the major peaks of fentanyl as a foundation, the mass of a substitution can be predicted by calculating the difference. For example, if 146 is not observed yet 160 is, subtracting 146 from 160 will indicate a substitution of a hydrogen by a methyl group. This way, differences in the mass units of the three main peaks within the spectra compared with those of fentanyl may be used to determine the mass of substitutions along the amide, aniline, or piperidine regions of the compound.
Pattern 4:
Cleavage at the amide C-N bond will generate the BP if a highly stabilized or highly substituted group is in the acyl region.
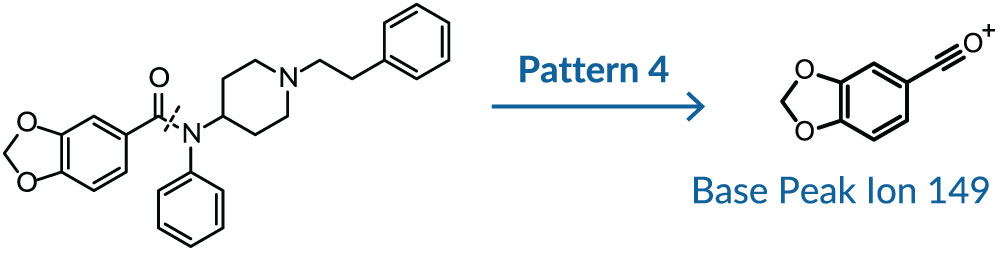
If the primary cleavage occurs along the amide C-N bond to generate the BP, instead of between the α/β site as for Pattern 1, then a highly resonance-stabilized group is likely substituted in the R1 position. This is the case for furanyl fentanyl (95) and benzodioxole fentanyl (149) (Pattern 4), as well as phenyl fentanyl (105) and thiophene fentanyl (111). Incidentally, cleavage also occurs at the α/β site for furanyl fentanyl and benzodioxole fentanyl, generating less abundant fragments 283 and 337, respectively (Figure 4).
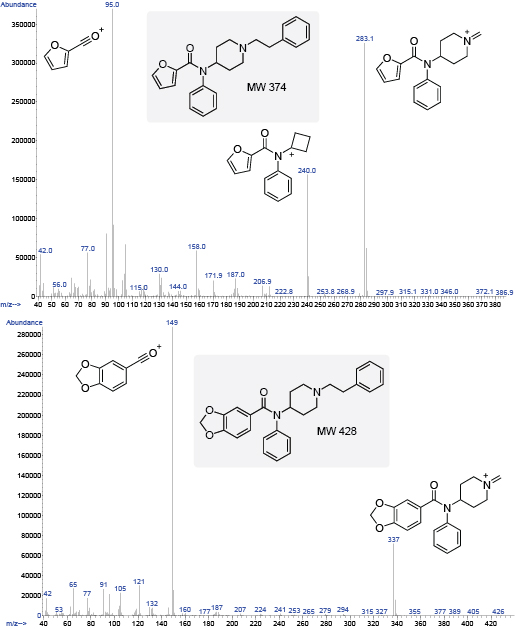
Figure 4. Cleavage at the amide C-N bond will generate the BP if a highly stabilized group has been substituted in the acyl region. The primary cleavage of the highly resonance-stabilized ion gives the BP fragments shown at 95 and 149 for furanyl fentanyl (left) and benzodioxole fentanyl (right).
It is also imperative to investigate the smaller molecular ion fragments to help identify your unknown because if the acyl amide group contains a different moiety such as a cyclopentyl (69), cyclobutyl (55), methoxyacetyl (45), or a tetrahydrofuran (71), these fragments will help you identify those moieties. For instance, with cyclopentyl fentanyl (Figure 3), there is a large fragment peak at 69, indicating cleavage occurs between the C-C bond next to the carbonyl group instead of the amide C-N bond as for furanyl fentanyl. For the most part BP ions are generated by primary cleavage at the α/β site.
Exceptions
While most fentanyl-type unknowns may be discerned using these predictive patterns, there are always a few exceptions to the rules. An exception occurs, for instance, when there is a substitution in the 4-position on the piperidine ring, such as with carfentanil. Pattern 1 still applies because the BP is shown at 303, yet the functional group in the 4-position is either eliminated during fragmentation or is retained, causing rearrangement leading to other fragments. Further investigation is needed for compounds similar to carfentanil that have substitutions in the 4-position on the piperidine ring. However, even the compounds that do not present all the predictive fragments will still display some areas of cleavage as outlined above.
In summary, by following the patterns outlined above (and taking into consideration notable exceptions), we hope that we have provided useful tips for interpreting the GC-MS of novel 4-anilidopiperidines. Please note that the fragment ion structures proposed in this article are predicted structures only and may not represent the true structures of the ions.
To cite this article: Pierzynski, H.G., Neubauer, L., Choi, C, et al. Tips for interpreting GC-MS fragmentation of unknown substituted fentanyls. Cayman Currents 28, 1-3 (2017).
References
1. Huang, B., Deutsche, K.H., Lalinde, N.L., et al. European Patent EP 0160422 B1 (1985).
2. Vardanyan, R.S. and Hruby, V.J. Future Med. Chem.6(4), 385-412 (2014).
3. Vučković, S., Prostran, M., Ivanović, M., et al. Curr. Med. Chem.16(19), 2468-2474 (2009).
4. Wang, L. and Bernert, J.T. J. Anal. Toxicol.30(5), 335-341 (2006).
5. Valaer, A.K., Huber, T., Andurkar, S.V., et al. J. Chromatogr. Sci.35(10), 461-466 (1997).
6. Higashikawa, Y. and Suzuki, S. Forensic Toxicol.26(1), 1-5 (2008).
7. Katselou, M., Papoutsis, I., Nikolaou, P., et al. Forensic Toxicol. 34(2), 201-212 (2016).
8. Ohta, H., Suzuki, S. and Ogasawara, K. J. Anal. Toxicol. 23(4), 280-285 (1999).
9. Breindahl, T., Kimergård, A., Andreasen, M.F., et al. Drug Test Anal.9(3), 415-422 (2017).
10. Goromaru, T., Katashima, M., Matsuura, H., et al. Chem. Pharm. Bull.33(9), 3922-3928 (1985).

