Stressed about Picking an Oxidative Damage Assay?
Stressed about Picking an Oxidative Damage Assay?
2022-11-30
Find the right biomarker to detect in your application
Oxidative stress occurs when there is an imbalance between the production of chemically reactive species such as reactive oxygen, nitrogen, and sulfur species (ROS, RNS, RSS) and antioxidant defense mechanisms. Uncontrolled oxidation can disrupt oxidation-reduction (redox) signaling and cause injury to cellular components like lipids, proteins, and nucleic acids, resulting in irreparable damage and eventual cell death.
Oxidative stress can be evaluated directly by measuring reactive species or indirectly by the associated damage to lipids, proteins, and nucleic acids. Although direct measurement of ROS is ideal, the indirect methods are often relied on more heavily due to the relative stability of damage markers on biomolecules compared to the transient nature of ROS.
Consider using one of Cayman's oxidative stress assays in your next experiment. Our assays rely on the tried-and-true techniques discussed below.
Download the Oxidative Stress: Markers & Detection Tools Guide
ROS
ROS are produced from molecular oxygen (O2) during normal cellular processes or in response to harmful exogenous factors (Figure 1). Various ROS including superoxide (O2•-), hydrogen peroxide (H2O2), and the hydroxyl radical (OH•) are formed, which can damage cellular macromolecules when uncontrolled.
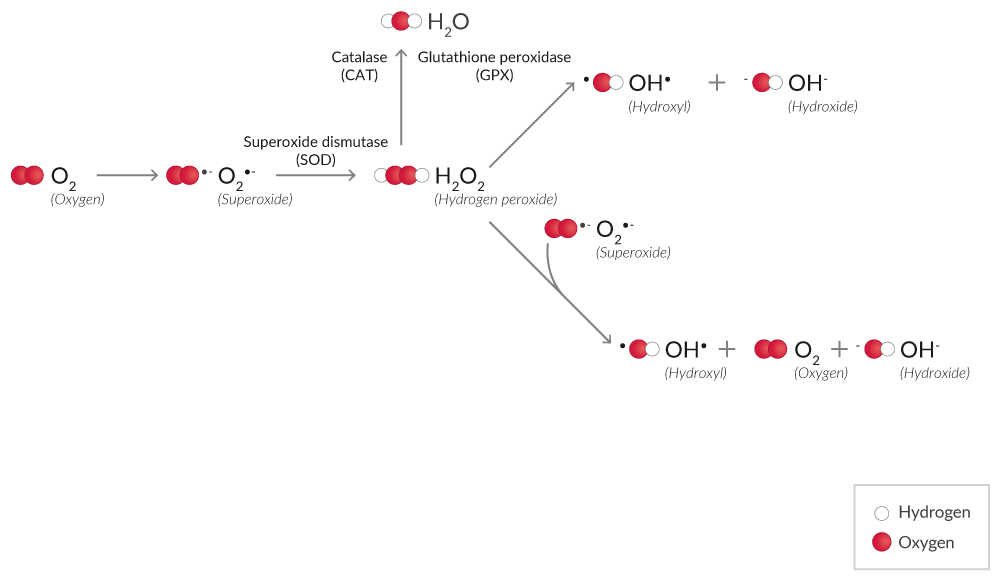
Figure 1. Simplified mechanism of ROS production from O2.
H2O2 is a less reactive ROS than other species.1 H2O2 assays using probes like ADHP (10-acetyl-3,7-dihydroxy phenoxazine) are widely used for the detection of H2O2.2 ADHP is colorless and non-fluorescent, but in the presence of peroxidase, ADHP reacts with H2O2, producing highly fluorescent resorufin.3 Assay specificity is improved significantly when an H2O2 scavenger, such as catalase, is included as a control.
Xanthine oxidase (XO), the terminal enzyme of purine catabolism (hypoxanthine → xanthine → uric acid) in humans, produces both H2O2 and O2•- as a byproduct of this reaction.4 XO activity assays utilize a multistep process where XO degrades hypoxanthine, and the H2O2 byproduct is captured with a probe like ADHP coupled to peroxidase.
ROS detection assays rely on redox-active fluorophores to measure ROS. Dihydroethidium is a widely used redox-sensitive probe that can be used in live cells.5,6 This probe is oxidized by O2•- to form 2-hydroxyethidium (Ex. 500-530 nm/Em. 590-620 nm) or by H2O2 or other ROS to form ethidium (Ex. 480 nm/Em. 576 nm). Note that the spectral range between 2-hydroxyethidium and ethidium is narrow, so O2•- can be distinguished from H2O2 with appropriate monochromator-based optical systems.
RNS
Reactive nitrogen species (RNS) are also produced during oxidative stress. High levels of O2•- and nitric oxide (NO•), synthesized by nitric oxide synthase (NOS), can lead to the formation of peroxynitrite (ONOO-), which reacts with protein tyrosine residues to form nitrotyrosine, a marker of protein nitration (Figure 2).7
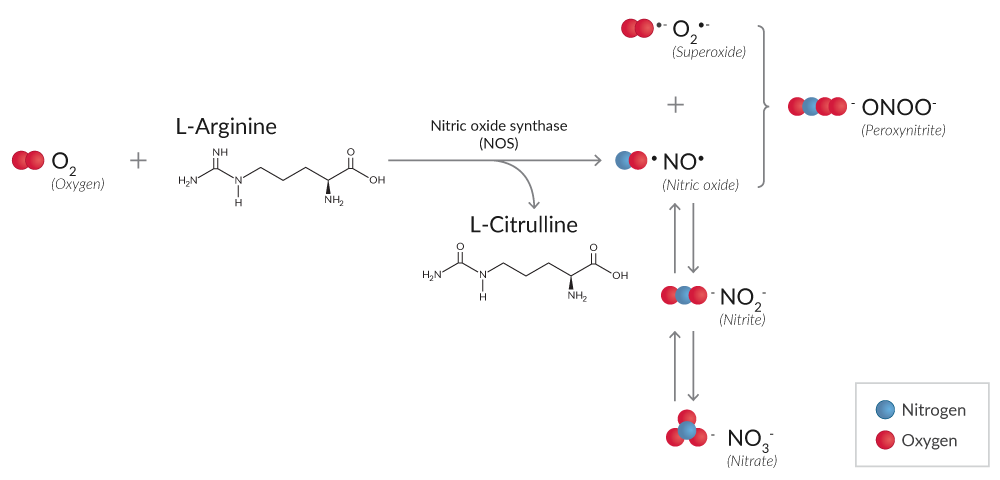
Figure 2. Simplified reaction mechanism of RNS production from O2 by NOS.
Nitrite (NO2-) and nitrate (NO3-) are degradation products of NO• reactions whose total production can be detected using either Griess reagents or DAN.8,9 Because the relative proportion of NO2- and NO3- is variable, NO2-/ NO3- assays first convert NO3- to NO2- using NADPH-dependent nitrate reductase. Subsequent reaction with Griess reagents or DAN, both of which only react with NO2-, will determine the total concentration of NO2-.
Detection of NOS activity in tissues and cells can be accomplished by harnessing the NOS-driven conversion of a radiolabeled arginine to citrulline in the presence of the necessary factors. Alternatively, in vitro NOS activity can be detected using the chemistry of the Griess reaction once the excess NADPH added as a cofactor for NOS activity is removed using an oxidization step that is catalyzed by lactate dehydrogenase (LDH).
| Related article: Fluorescent and Luminescent Probes for ROS, RNS, Thiols, and Metal Ions |
Protein Oxidation
Oxidative stress can result in oxidative modifications to both the protein backbone and side chain amino acids (Figure 3).
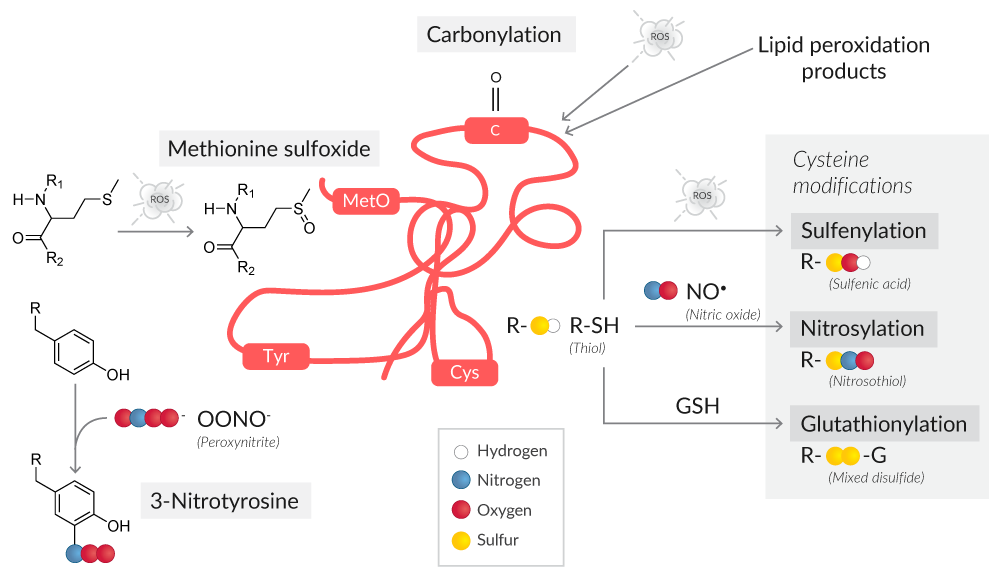
Figure 3. Overview of protein oxidative modifications as a result of ROS or RNS.
Protein carbonyl content is the most common marker of protein oxidation.10 The presence of redox cycling cations like Fe2+ or Cu2+, in conjunction with attack by ROS, leads to the formation of amino acid derivatives containing carbonyl groups (ketones, aldehydes).
The amino acids most susceptible to oxidative stress are cysteine and methionine, with cysteine being more vulnerable because of its ionizable thiol group.11 Protein oxidation at cysteine thiol groups can result in oxidative post-translational modifications like S-sulfenylation, S-nitrosylation, and S-glutathionylation, all of which can alter protein structure and/or function.12
Thiol detection assays are performed with fluorometric probes that react with thiol groups, emitting a fluorescence signal that is proportional to the free thiol content in samples.
S-Nitrosylated protein assays and S-glutathionylated protein assays commonly use the biotin switch method for the detection of these oxidative modifications. This technique starts with blocking existing free thiols, followed by a reducing step where the oxidative modifications are converted to new free thiols.13 The newly formed thiols are then biotinylated, and the biotinylated proteins can be localized or purified with avidin-coupled reagents.
| Related article: Chemical Probes for Detecting Endogenous Protein S-Nitrosation |
S-Sulfenylated proteins have been historically assayed with methods like GC/MS, HPLC, Western blotting, and ELISA.14 However, such methods are cell destructive, obscuring the localization of sulfenylated proteins and their physiological and pathological roles in living cells. Assays for sulfenylated proteins performed with DAz-2, a cell-permeable and chemoselective probe for sulfenic acids, use a two-step reaction to detect sulfenic acid-modified proteins in situ. First, sulfenylated proteins are labeled with DAz-2, which is then conjugated to phosphine-biotin and analyzed with avidin-coupled reagents.15
Oxidative stress to a protein's methionine residues generates protein methionine sulfoxide (MetO), a reversible oxidative modification that if not corrected by MetO reductases is irreversibly oxidized to methionine sulfone.16 To detect proteins with MetO residues by Western blot, antibodies specific for protein methionine sulfoxide can be used.
Lipid Peroxidation
Lipid peroxidation results in the formation of highly reactive and unstable lipid hydroperoxides (LPOs) of unsaturated lipids and their degradation products (Figure 4).
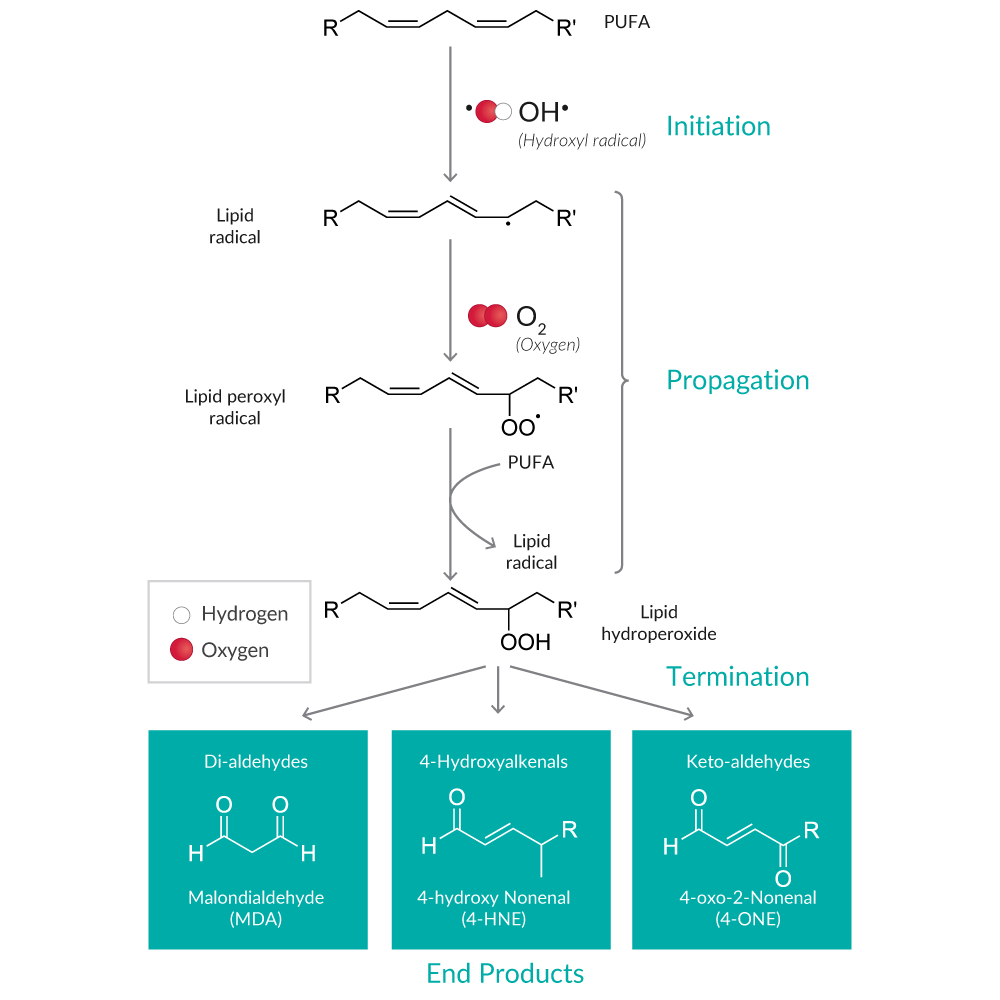
Figure 4. Overview of the lipid peroxidation pathway and major end products.
One method for determining the extent of lipid peroxidation is to measure LPOs directly. LPO assays first extract LPOs into chloroform, a step that eliminates interference in the assay caused by H2O2 or endogenous ferric ions. Then, the total LPO content is determined by directly utilizing redox reactions between LPOs and ferrous ions.
LPO decomposition products (e.g., alkanes, ketones, aldehydes) are more commonly used as markers of lipid peroxidation because they are far more stable.17 Malondialdehyde (MDA) and 4-hydroxy nonenal (4-HNE) are the most well-known degradants of polyunsaturated fatty acid (PUFA) LPOs. MDA assays using a thiobarbituric acid (TBA) reaction are named Thiobarbituric Acid Reactive Substances assays or TBARS assays, since TBA reacts with other aldehydes produced during lipid peroxidation in addition to MDA. While the specificity of TBARS toward compounds other than MDA generates uncertainty, it remains the most widely used assay used to determine lipid peroxidation.18
Assays to quantitate 1,4-dihydroxynonane mercapturic acid (DHN-MA), the major urinary metabolite of 4-HNE, is an additional analysis that can be performed as a marker of lipid peroxidation. It should be noted that measurement of 4-HNE as an indirect indicator of lipid peroxidation will not provide a complete analysis since only LPOs derived from ω-6 PUFAs (and not ω-3 PUFAs) give rise to this product.19
| Related article: Ferroptosis: Detecting a New Form of Cell Death |
8-Isoprostane, produced by random oxidation of tissue phospholipids, is considered one of the most reliable markers of in vivo lipid peroxidation.20 It is a specific and stable product of lipid peroxidation: 8-isoprostane is esterified in membrane phospholipids and/or present in a free form in detectable quantities in all normal biological fluids and tissues.21 Most of the 8-isoprostane in tissues (as well as plasma and serum) is esterified with lipids, so hydrolysis must be performed if determination of total (esterified and free) tissue 8-isoprostane is desired. Measurement of 8-isoprostane in urine will assess levels of the free form.
8-isoprostane immunoassays and LC-MS or GC-MS are typically used to assess 8-isoprostane levels. Note that it is common for immunoassays to report higher analyte concentrations compared to LC-MS or GC-MS. With the appropriate 8-isoprostane analytical standards, LC-MS or GC-MS analyses typically measure only a single compound, whereas antibodies used in immunoassays can be cross-reactive with structurally similar isoprostanes.
| Related article: The Mechanism of Isoprostane Formation |
DNA/RNA Damage
Oxidative stress can damage DNA and RNA, resulting in nucleobase lesions, double- and single-strand breaks, and inter- and intra-strand crosslinks, among other damage markers (Figure 5). Guanine is the base most prone to oxidation when DNA and RNA are damaged.22 The repair processes that are initiated to correct this damage result in the release of multiple oxidized guanine species into the urine. These species include the ribose-free base (8-hydroxyguanine), the nucleoside from RNA (8-hydroxyguanosine), and the deoxynucleoside from DNA (8-hydroxy-2'-deoxyguanosine).
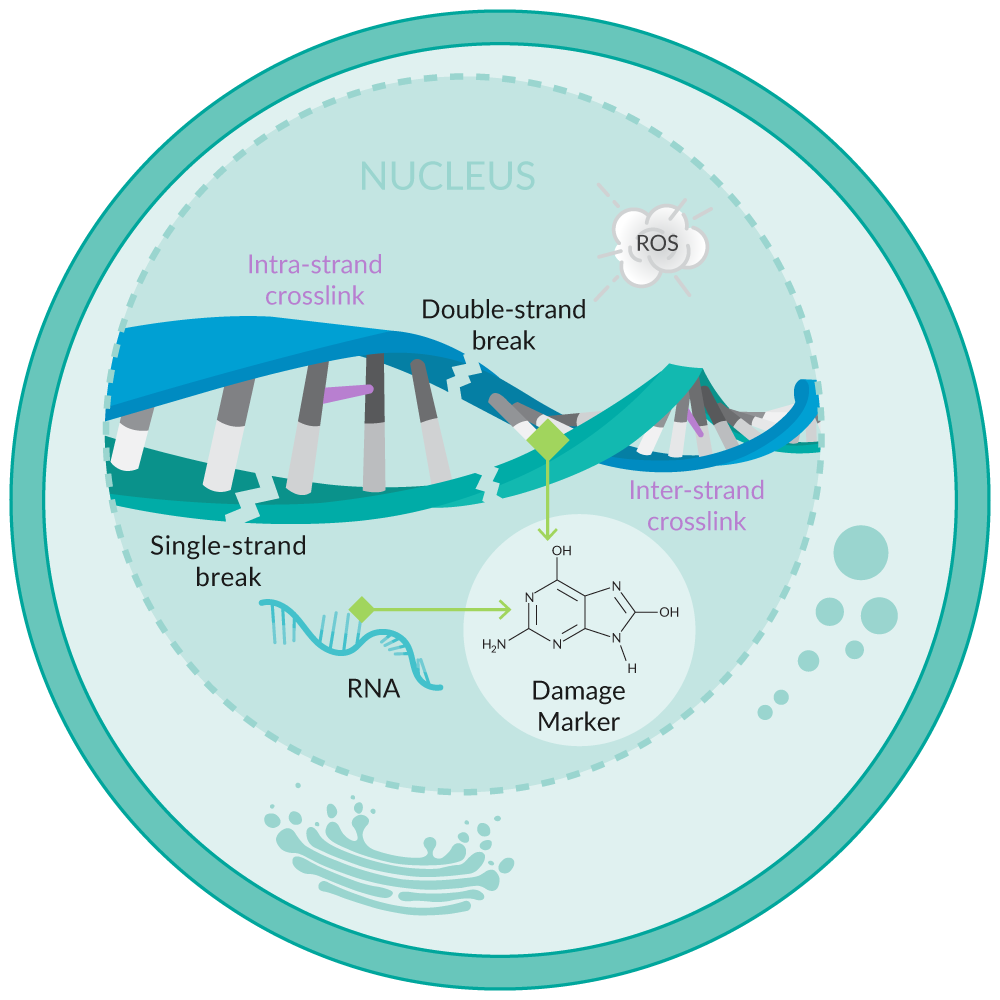
Figure 5. Overview of oxidative stress-induced DNA/RNA damage.
Assays that detect multiple oxidized guanine species capture a more complete set of biologically relevant products of oxidative damage than assays that are restricted to analysis of only one (e.g., 8-hydroxy-2'-deoxyguanosine).
| Related article: Detecting DNA/RNA Damage: A comparison of Cayman's DNA/RNA Damage ELISAs |
Cellular Antioxidants Protect Against Oxidative Stress
Enzymatic antioxidants protect vital cellular functions by scavenging reactive species and preventing irreversible injury. Quantification of these enzyme activities is essential in the characterization of the antioxidant capabilities of a biological system.
ROS Detoxifying Enzymes
Some enzymatic antioxidants like superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPX) eliminate ROS, preventing their accumulation and the propagation of further oxidation and cell injury (Figure 6).1
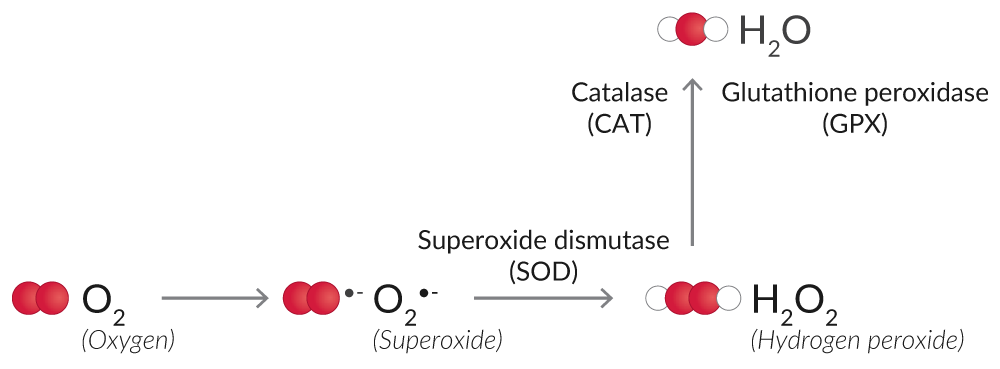
Figure 6. Overview of enzymatic antioxidants that eliminate ROS.
SOD converts O2•- into the less reactive H2O2. SOD activity assays measure the dismutation of O2•- with a tetrazolium salt, which is converted to a formazan dye. Cytosolic and mitochondrial SOD can be assayed together as a measurement of total SOD activity, or the activity of SOD
| Related article: Measure Mitochondrial H2O2 Directly In Vivo or in Cells |
Catalase and GPX catalyze the conversion of H2O2 to O2 and H2O, thus halting the accumulation of ROS.
Catalase has both catalytic activity (2 H2O2 → O2 + 2 H2O) and peroxidatic activity (H2O2 + AH2 → A + 2 H2O, where A is an aliphatic alcohol).23 Aliphatic alcohols are a substrate specific for catalase; other enzymes with peroxidatic activity do not utilize them. Hence, catalase activity assays relying on the peroxidatic activity of catalase are specific for this enzyme.
GPX uses reduced glutathione (GSH) to degrade H2O2 to O2.24 Glutathione is a tripeptide and major cellular antioxidant that is oxidized (GSSG) to eliminate H2O2 or repair oxidized proteins.25 Glutathione reductase (GR) catalyzes the NADPH-dependent reduction of oxidized glutathione (GSSG) to replenish GSH. GPX activity assays rely on these coupled enzymatic reactions for the determination of GPX activity, and GR activity assays measure the rate of NADPH oxidation to determine GR activity.
A high GSH:GSSG ratio is essential for protection against oxidative stress, and the ratio of GSH:GSSG is frequently used a marker of oxidative stress.26 Glutathione assays can be used to measure total glutathione, GSH, and/or GSSG.
Redox Regulation
Redox reactions are a critical component of cell signaling pathways and cellular metabolism but can result in oxidative stress if left unchecked.27 Reducing agents like GSH and NADPH donate electrons to oxidized acceptors like GSSG or protein disulfides, restoring them to their reduced state. The glutaredoxin (Grx) and thioredoxin (Trx) systems harness these reducing agents to balance cellular redox status.
Grx catalyzes the deglutathionylation of glutathionylated proteins and the reduction of protein disulfides to regulate redox signal transduction and repair oxidized proteins (Figure 7).25 Oxidized Grx is reduced by GSH, forming GSSG, which is reduced back to GSH by GR at the expense of NADPH.
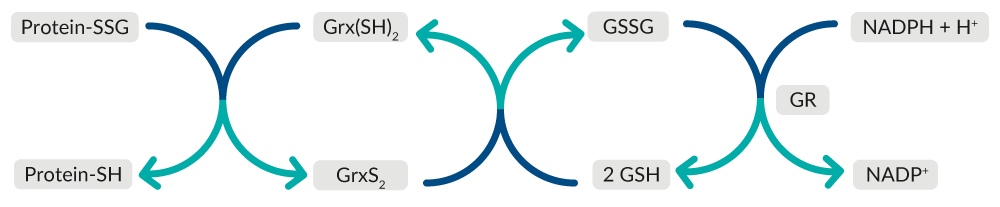
Figure 7. Overview of Grx-mediated repair of oxidized proteins.
Trx catalyzes the reduction of protein disulfides, maintaining cellular thiol redox homeostasis (Figure 8). Oxidized Trx is restored to its reduced state by thioredoxin reductase (TrxR) at the expense of NADPH.
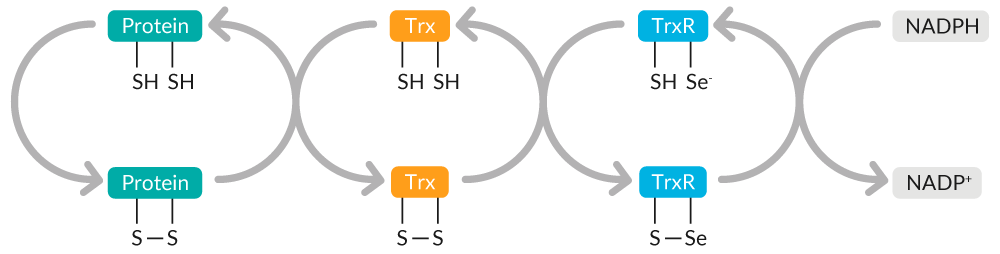
Figure 8. Overview of Trx-mediated maintenance of cellular thiol redox homeostasis.
The endogenous reactions catalyzed by Grx and Trx form the basis for their activity assays. Grx activity assays and Trx activity assays use coupled enzymatic assays. In these assays, the reaction catalyzed by the enzyme of interest is paired with a second enzymatic reaction, yielding a detectable product that is proportional to the activity of the enzyme of interest.
TrxR activity assays use a more direct approach, measuring TrxR activity by monitoring the NADPH-dependent reduction of DTNB, also known as Ellman's reagent, to TNB.
Scavenging Assays for Antioxidant Activity
ABTS or DPPH radicals can be used in scavenging assays to measure the antioxidant capacity of a compound or sample. ABTS is a radical cation and a substrate of peroxidases.28 It has a blue color in the presence of sodium persulfate or metmyoglobin but decolorizes upon incubation with antioxidants.29,30 ABTS has commonly been used to assess antioxidant capacity in Trolox equivalent antioxidant capacity (TEAC) assay, where the capacity of antioxidants in the sample are compared to the reference antioxidant Trolox. Similarly, DPPH turns from deep violet to yellow upon reactions with antioxidants.31 The color changes of ABTS and DPPH can be measured spectrophotometrically.
The cooperation among different antioxidants provides greater protection against attack by reactive species than any single compound alone. Assays that evaluate the overall antioxidant capacity may provide more meaningful biological information compared to that obtained by the measurement of individual components, as it considers the cumulative effect of all antioxidants present.
Concluding Remarks
The quantification of reactive species, the damage produced, and cellular antioxidant defense mechanisms is critical for determining the extent of oxidative injury. With a complete understanding of the techniques used to detect the most common markers of oxidative damage, you can be confident that you are choosing the right tool for your application.
Next Steps
| Assess the ultimate consequence of oxidative injury in your system with a cell death assay - this guide can help you determine the cell death mechanism(s) occurring in your experiment. |  |
Can't find the right assay? Let's talk.
Contact a technical support scientist or inquire about custom assay development.
Oxidative Stress Assays At-A-Glance
ROS
| Item No. | Product Name | Sample Type | Measure | Methodology | Additional Info |
|---|---|---|---|---|---|
| 600050 | Hydrogen Peroxide Assay Kit | Cultured cells | Extracellular H2O2 | Plate-based fluorometric measurement (ex 530-560 nm, em 590 nm) | Utilizes ADHP, a sensitive and stable probe for H2O2 and includes catalase to check assay specificity |
| 701600 | Mitochondrial ROS Detection Assay Kit | Viable Cells | Mitochondrial ROS | Fluorescence, flow cytometer, or plate-based fluorometric measurement (Ex. 480-515 nm, Em. 560-600 nm) | When properly titrated, the Mitochondrial ROS Detection Reagent accumulates in the mitochondrial matrix and fluoresces in the presense of ROS |
| 780001 | Nitrate/Nitrite Colorimetric Assay Kit | Plasma, serum, urine, tissue culture media, and tissue homogenates | NO• metabolites | Plate-based colorimetric measurement (540-550 nm) | Uses a small amount of added NADPH in conjunction with a catalytic system for recycling spent NADP+ back to NADPH to avoid NADPH interference with the chemistry of the Griess reagents; works well for the analysis of fluids such as plasma and urine, but cannot be used to analyze NO2- and NO3- from an in vitro assay of NOS in which excess NADPH has been added |
| 760871 | Nitrate/Nitrite Colorimetric Assay Kit (LDH method) | Plasma, serum, urine, and tissue homogenates | In vitro NOS activity and NO• metabolites | Plate-based colorimetric measurement (530-550 nm) | Use to analyze NO2- and NO3- from an in vitro NOS assay in which excess NADPH has been added; an extra step is included in the protocol that uses LDH to remove the excess NADPH |
| 780051 | Nitrate/Nitrite Fluorometric Assay Kit | Plasma, serum, tissue culture media, and tissue homogenates | NO• metabolites | Plate-based fluorometric measurement (ex 360-365 nm, em 430 nm) | Utilizes DAN instead of Griess reagents, which enables 20-fold increased sensitivity over the colorimetric version; allows for detection of low concentrations of NO2- and NO3- (minimum detectable quantity of NO2/NO3 is ~50 nM) |
| 781001 | NOS Activity Assay Kit | Cell lysates and purified preparations | NOS activity | Liquid scintillation counting | Monitors the conversion of radiolabeled arginine to citrulline by NOS |
| 601290 | ROS Detection Cell-Based Assay Kit (DHE) | Live cells | ROS | Flow cytometer or plate-based fluorometric measurement (ex 480-520 nm, em 570-600 nm) | Utilizes the redox-sensitive probe DHE as a substrate for O2•− and H2O2; includes positive control for ROS generation and negative control for ROS scavenging |
| 10010895 | Xanthine Oxidase Fluorometric Assay Kit | Plasma, serum, and tissue homogenates | XO activity | Plate-based fluorometric measurement (ex 520-550 nm, em 585-595 nm) | Based on a multistep enzymatic reaction in which the H2O2 produced when XO oxidizes hypoxanthine reacts with ADHP |
RNS
| Item No. | Product Name | Sample Type | Measure | Methodology | Additional Info |
|---|---|---|---|---|---|
| 780001 | Nitrate/Nitrite Colorimetric Assay Kit | Plasma, serum, urine, tissue culture media, and tissue homogenates | NO• metabolites | Plate-based colorimetric measurement (540-550 nm) | Uses a small amount of added NADPH in conjunction with a catalytic system for recycling spent NADP+ back to NADPH to avoid NADPH interference with the chemistry of the Griess reagents; works well for the analysis of fluids such as plasma and urine, but cannot be used to analyze NO2- and NO3- from an in vitro assay of NOS in which excess NADPH has been added |
| 760871 | Nitrate/Nitrite Colorimetric Assay Kit (LDH method) | Plasma, serum, urine, and tissue homogenates | In vitro NOS activity and NO• metabolites | Plate-based colorimetric measurement (530-550 nm) | Use to analyze NO2- and NO3- from an in vitro NOS assay in which excess NADPH has been added; an extra step is included in the protocol that uses LDH to remove the excess NADPH |
| 780051 | Nitrate/Nitrite Fluorometric Assay Kit | Plasma, serum, tissue culture media, and tissue homogenates | NO• metabolites | Plate-based fluorometric measurement (ex 360-365 nm, em 430 nm) | Utilizes DAN instead of Griess reagents, which enables 20-fold increased sensitivity over the colorimetric version; allows for detection of low concentrations of NO2- and NO3- (minimum detectable quantity of NO2/NO3 is ~50 nM) |
| 781001 | NOS Activity Assay Kit | Cell lysates and purified preparations | NOS activity | Liquid scintillation counting | Monitors the conversion of radiolabeled arginine to citrulline by NOS |
Protein Modifications
| Item No. | Product Name | Sample Type | Measure | Methodology | Additional Info |
|---|---|---|---|---|---|
| 600160 | Methionine Sulfoxide Immunoblotting Kit | Plasma, CSF, cell/tissue lysates, or semi-pure/purified proteins | Proteins containing MetO residues | Immunochemical detection by Western blot | Utilizes a MetO polyclonal antibody isolated from rabbit serum that is specific for MetO and demonstrates minimal cross-reactivity with methionine sulfone |
| 10010721 | S-Glutathionylated Protein Detection Kit | Whole (permeabilized) cells | Protein-PSSG adducts | Streptavidin-based detection by flow cytometry, fluorescence microscopy, or IP/avidin overlay analysis | Utilizes a modified 'Biotin-switch' method to directly tag protein-PSSG adducts |
| 10006518 | S-Nitrosylated Protein Detection Kit (Biotin Switch) | Whole cells or tissues | S-NO proteins | Streptavidin-based detection by Western blot or fluorescence microscopy | Utilizes a modified 'Biotin-switch' method to directly tag S-NO proteins |
| 601220 | Nitrotyrosine IP Kit | Cell lysates | Nitrated tyrosine content | Affinity sorbent capture and elution by Western blot or proteomic analysis | Utilizes a sorbent coupled with a nitrotyrosine monoclonal antibody to capture and pulldown nitrated proteins |
| 10005020 | Protein Carbonyl Colorimetric Assay Kit | Plasma, serum, urine, tissue homogenates, and cell lysates | Protein carbonyl content | Plate-based colorimetric measurement (360-385 nm) | Utilizes the reaction between DNPH and protein carbonyls as a readout of protein oxidation |
| 700340 | Thiol Detection Assay Kit | Plasma, serum, urine, cell lysates, and tissue homogenates | Free thiol content | Plate-based fluorometric measurement (Ex. 380-390 nm, Em. 510-520 nm) | Fluorometric detector reacts with free cysteine, glutathione, and cysteine residues on proteins to emit a fluorescent signal as a readout of thiol content |
Lipid Peroxidation
| Item No. | Product Name | Sample Type | Measure | Methodology | Additional Info |
|---|---|---|---|---|---|
| 501140 | DHN-MA EIA Kit | Urine | DHN-MA, a 4-HNE metabolite | Plate-based colorimetric measurement (405-414 nm) | Compatible with human, mouse, rat, dog, and pig samples |
| 516351 | 8-Isoprostane ELISA Kit | Plasma, serum, urine, lavage fluids, tissue homogenates, and culture medium | 8-Isoprostane | Plate-based colorimetric measurement (405-420 nm) | Overnight assay (incuabtion time = 18 hours) uses AChE tracer; assay range 0.8-500 pg/ml; detection limit (80% B/B0) of ~ 3 pg/ml |
| 516360 | 8-Isoprostane Express ELISA Kit | Plasma, serum, urine, lavage fluids, tissue homogenates, and culture medium | 8-Isoprostane | Plate-based colorimetric measurement (405-420 nm) | 4 hour assay uses AChE tracer; assay range 2.5-1,500 pg/ml; detection limit (80% B/B0) of ~ 10 pg/ml |
| 500431 | STAT-8-Isoprostane ELISA Kit | Plasma, serum, urine, lavage fluids, tissue homogenates, and culture medium | 8-Isoprostane | Plate-based colorimetric measurement (405-420 nm) | Extremely rapid assay (results in ~2 hours) uses an AP tracer; assay range 23.4-3,000 pg/ml; detection limit (80% B/B0) of ~ 45 pg/ml |
| 705002 | Lipid Hydroperoxide (LPO) Assay Kit | Tissues, cultured cells, plant materials, foods, and biological fluids | LPOs | Plate-based colorimetric measurement (500 nm) | Designed for use with a single-tube spectrophotometer to read the results |
| 705003 | Lipid Hydroperoxide (LPO) Assay Kit (96 well) | Tissues, cultured cells, plant materials, foods, and biological fluids | LPOs | Plate-based colorimetric measurement (500 nm) | Designed for use with a reusable glass plate |
| 10009055 | TBARS Assay Kit | Plasma, serum, urine, tissue homogenates, and cell lysates | MDA-TBA adduct | Plate-based colorimetric measurement (530-540 nm) or fluorometric measurement (ex 530 nm, em 550 nm) | Standard method to determine lipid peroxidation; reaction yields higher sensitivity when measured fluorometrically, but a colorimetric method is included as an option |
| 700870 | TBARS (TCA Method) Assay Kit | Plasma, serum, urine, tissue homogenates, and cell lysates | MDA-TBA adduct | Plate-based colorimetric measurement (530-540 nm) or fluorometric measurement (ex 530 nm, em 550 nm) | Offers the advantage of improved sample processing and reduced working volumes by incorporating a TCA precipitation procedure; maintains same reliability and accuracy as the original TBARS Assay; includes sample acid precipitation protocol to avoid confounding soluble TBARS |
DNA/RNA Damage
| Item No. | Product Name | Sample Type | Measure | Methodology | Additional Info |
|---|---|---|---|---|---|
| 589320 | DNA/RNA Oxidative Damage ELISA Kit | Urine, cell culture medium, cell lysates, tissue samples, saliva, and plasma/serum samples | 8-hydroxy-2’-deoxyguanosine, 8-hydroxy guanosine, and 8-hydroxy guanine | Plate-based colorimetric measurement (405-420 nm) | Monoclonal antibody (clone 15A3) used enables detection of 8-hydroxy-2’-deoxyguanosine (DNA oxidative damage marker), 8-hydroxy guanosine (RNA damage marker), and 8-hydroxyguanine (DNA/RNA damage marker) with selectivity and sensitivity highest for 8-hydroxy-2’-deoxyguanosine; correlates with LC/MS measurements of 8-hydroxy-2'-deoxyguanosine, though high slope indicates other unknown species are detected |
| 501130 | DNA/RNA Oxidative Damage (Clone 7E6.9) ELISA Kit | Urine (other sample types have not been validated but may be used) | 8-hydroxy-2’-deoxyguanosine and 8-hydroxy guanosine | Plate-based colorimetric measurement (405-420 nm) | Monoclonal antibody (clone 7E6.9) used enables detection of 8-hydroxy-2’-deoxyguanosine (DNA oxidative damage marker) and 8-hydroxyguanosine (RNA damage marker) with equal selectivity and sensitivity; correlates with LC/MS measurements of a combination of 8-hydroxy-2'-deoxyguanosine and 8-hydroxy guanine |
Antioxidant Detection
| Item No. | Product Name | Sample Type | Measure | Methodology | Additional Info |
|---|---|---|---|---|---|
| 709001 | Antioxidant Assay Kit | Plasma, serum, urine, saliva, or cell lysates | Total antioxidant capacity | Plate-based colorimetric measurement (750 or 405 nm) | Designed to measure the cumulative effects of all antioxidants present in plasma and body fluids, which offers more complete biological information compared to that obtained by the assessment of individual antioxidants |
| 700420 | Ascorbate Assay Kit | Plasma, serum, urine, and fruit juices | Ascorbate (L-ascorbic acid/vitamin C) | Plate-based fluorometric measurement (ex 340-350 nm, em 420-430 nm) | Utilizes the condensation reaction of dehydroascorbic acid, the oxidation product of ascorbate, to determine ascorbate concentration |
| 707002 | Catalase Assay Kit | Plasma, serum, erythrocyte lysates, tissue homogenates, and cell lysates | Catalase activity | Plate-based colorimetric measurement (540 nm) | Utilizes the peroxidatic function of catalase to determine enzyme activity |
| 700910 | Catalase Assay Kit (without Hydrogen Peroxide) | Plasma, serum, erythrocyte lysates, tissue homogenates, and cell lysates | Catalase activity | Plate-based colorimetric measurement (540 nm) | The 30% H2O2 solution needed to perform this assay is not included in this kit version as a shipping consideration to countries/institutions that regulate the receipt of such substances |
| 600360 | Glutathione Cell-Based Detection Kit (Blue Fluorescence) | Cell lysates | Intracellular GSH levels | Plate-based fluorometric measurement (ex 380 nm, em 480 nm) | Utilizes a cell-permeable dye that reacts with GSH to generate a fluorescent product that indicates intracellular level of GSH |
| 703202 | Glutathione Reductase Assay Kit | Plasma, cell lysates, and tissue homogenates | GR activity | Plate-based colorimetric measurement (340 nm) | Measures the rate of NADPH oxidation as a readout of GR activity |
| 500239 | Glutaredoxin Fluorometric Activity Assay Kit | Cell lysates and tissue samples | Grx activity | Plate-based fluorometric measurement (Ex. 520 nm, Em. 560 nm) | The substrate eosin-GS-BSA is reduced by Grx, giving the fluorescent product eosin-GSH, which is used as a readout of Grx activity |
| 706002 | Superoxide Dismutase Assay Kit | Plasma, serum, tissue homogenates, and cell lysates | Cu/Zn, Mn, and Fe SOD activity | Plate-based colorimetric measurement (440-460 nm) | Measures the dismutation of superoxide radicals generated by XO and hypoxanthine as a readout of the activity of all three types of SOD |
| 500228 | Thioredoxin Fluorometric Activity Assay Kit | Cell lysates | Trx activity | Plate-based fluorometric measurement (Ex. 520, Em. 560 nm) | Measures Trx activity by monitoring the reduction of eosin-labeled insulin disulfides by Trx |
| 10007892 | Thioredoxin Reductase Colorimetric Assay Kit | Tissues homogenates and cell lysates | TrxR activity | Plaste-based colorimetric measurement (405-414 nm) | Monitors TrxR-mediated reduction of DTNB to TNB as a readout of TrxR activity |
References
1. Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 20(10), 2407 (2019).
2. Lü, J.-M., Lin, P.H., Yao, Q., et al. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 14(4), 840-860 (2010)
3. Zhou, M., Diwu, Z., Panchuck-Voloshina, N., et al. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: Applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 253(2), 162-168 (1997).
4. Kelley, E.E., Khoo, N.K.H., Hundley, N.J., et al. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic. Biol. Med. 48(4), 493-498 (2010).
5. Michalski, R., Michalowski, B., Sikora, A., et al. On the use of fluorescence lifetime imaging and dihydroethidium to detect superoxide in intact animals and Ex. vivo tissues: A reassessment. Free Radic. Biol. Med. 67, 278-284 (2014).
6. Zhao, H., Kalivendi, S., Zhang, H., et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: Potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 34(11), 1359-1368 (2003).
7. Mishra, D., Patel, V., and Banerjee, D. Nitric oxide and s-nitrosylation in cancers: Emphasis on breast cancer. Breast Cancer (Auckl) 14, 1178223419882688 (2020).
8. Green, L.C., Wagner, D.A., Glogowski, J., et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126(1), 131-138 (1982).
9. Misko, T.P., Schilling, R.J., Salvemini, D., et al. A fluorometric assay for the measurement of nitrite in biological samples. Anal. Biochem. 214(1), 11-16 (1993).
10. Stadtman, E.R. and Oliver, C.N. Metal-catalyzed oxidation of proteins. Physiological consequences. J. Biol. Chem. 266(4), 2005-2008 (1991).
11. Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 80, 148-157 (2015).
12. Baba, S.P. and Bhatnagar, A. Role of thiols in oxidative stress. Curr. Opin. Toxicol. 7, 133-139 (2018).
13. Forrester, M.T., Foster, M.W., Benhar, M., et al. Detection of protein S-nitrosylation with the biotin-switch technique. Free Radic. Biol. Med. 46(2), 119-126 (2009).
14. Hawkins, C.L., Morgan, P.E., and Davies, M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 46(8), 965-988 (2009).
15. Leonard, S.E., Reddie, K.G., and Carroll, K.S. Mining the thiol proteome for sulfenic acid modifications reveals new targets for oxidation in cells. ACS Chem. Biol. 4(9), 783-799 (2009).
16. Agbas, A. and Moskovitz, J. The role of methionine oxidation/reduction in the regulation of immune response. Curr. Signal Transduct. Ther. 4(1), 46-50 (2009).
17. Ayala, A., Muñoz, M.F., and Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 360438 (2014).
18. Khoubnasabjafari, M., Ansarin, K., and Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. Bioimpacts 5(3), 123-127 (2015).
19. Zhong, H. and Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 4, 193-199 (2015).
20. Roberts, L.J. and Morrow, J.D. Measurement of F2-isoprostanes as an indEx. of oxidative stress in vivo. Free Radic. Biol. Med. 28(4), 505-513 (2000).
21. Milne, G.L., Yin, H., Hardy, K.D., et al. Isoprostane generation and function. Chem. Rev. 111(10), 5973-5996 (2011).
22. Kino, K., Hirao-Suzuki, M., Morikawa, M., et al. Generation, repair and replication of guanine oxidation products. Genes Environ. 39, 21 (2017).
23. Wheeler, C.R., Salzman, J.A., Elsayed, N.M., et al. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal. Biochem. 184(2), 193-199 (1990).
24. Curieses Andrés, C.M., Pérez de la Lastra, J.M., Juan, C.A., et al. Chemistry of hydrogen peroxide formation and elimination in mammalian cells, and its role in various pathologies. Stresses 2(3), 256-274 (2022).
25. Ribas, V., García-Ruiz, C., and Fernández-Checa, J.C. Glutathione and mitochondria. Front. Pharmacol. 5, 151 (2014).
26. Baker, M.A., Cerniglia, G.J., and Zaman, A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem. 190(2), 360-365 (1990).
27. Muri, J. and Kopf, M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 21(6), 363-381 (2021).
28. Bhattarai, H.D., Paudel, B., Lee, H.S., et al. Antioxidant activity of Sanionia uncinata, a polar moss species from King George Island, Antarctica. Phytother. Res. 22(12), 1635-1639 (2008).
29. Huang, D., Ou, B., and Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 53(6), 1841-1856 (2005).
30. Walker, R.B. and Everette, J.D. Comparative reaction rates of various antioxidants with ABTS radical cation. J. Agric. Food Chem. 57(4), 1156-1161 (2009).
31. Molyneux, P. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 26(2), 211-219 (2004).


