Molecular Chaperones in Control of the Heat Shock Response
Molecular Chaperones in Control of the Heat Shock Response
2017-11-27
Heat shock response is an immediate and highly conserved reprogramming of cellular activities in riposte to stress. It involves the rapid synthesis of a battery of cytoprotective heat shock proteins (Hsps) and the post-transcriptional inhibition of normal protein synthesis to regulate the folding and remodeling of proteins. By repairing and adapting to damage caused by stress, this fundamental pathway confers tolerance to future cellular injuries and reduces cell death. Though Hsps are generally responsible for remediating damage to proteins in response to high levels of heat, they may also be induced by environmental stressors such as oxidants, viral infection, heavy metal exposure, ethanol consumption, amino acid analogs, ischemia, and disruptions to energy metabolism. Under normal conditions, Hsps also participate in nascent protein synthesis with a necessary role in quality control.
Heat shock proteins are classified and named according to their apparent molecular mass: small Hsp family (~20 kDa), Hsp40 family (~40 kDa), Hsp60 family (~60 kDa), Hsp70 family (~70 kDa), Hsp90 family (~90 kDa), and Hsp110 family (~110 kDa). As molecular chaperones, they cooperate in multiprotein and multicomplex networks to protect damaged proteins from aggregation, unfold aggregated proteins, and refold damaged proteins or target them for efficient degradation. Current research efforts are focused on a better understanding of how the various components of the heat shock response work together, with the goal to more effectively target Hsps therapeutically in disorders of protein homeostasis and folding. To enable this area of research, Cayman carries active, pure recombinant Hsps and co-chaperones as well as inhibitors to many of the major Hsps and antibodies to detect heat shock transcription factors. These products are highlighted throughout this review.
Cytoplasmic Chaperones
Hsp70 and co-factors
Hsp70
Hsp70 is thought to be the most highly conserved family of proteins throughout evolution. This stress-induced chaperone ensures proper folding of nascent peptides or misfolded proteins by recognizing elements in the tertiary structure of client proteins, usually exposed hydrophobic patches that are typically buried in a properly folded protein, and delivering those clients to Hsp90 for final maturation. It consists of two functional domains: A C-terminal protein substrate-binding domain and an N-terminal ATP-binding domain (Figure 1). The substrate-binding domain consists of two subdomains, a two-layered β-sandwich subdomain, which contains the peptide binding pocket, and an α-helical subdomain, which serves as a lid to cover the substrate binding cleft. The ATP-binding domain consists of four subdomains split into two lobes by a central ATP/ADP binding pocket. The two terminal domains are connected by an interdomain linker, which is critical for allosteric communication between domains. A variable region (EEVD sequence) at the very end of the C-terminal domain acts as a docking site for co-chaperones with the help of accessory proteins. Hsp70 binds to short hydrophobic stretches of nascent or unfolded polypeptides through the substrate-binding domain in an ATP-dependent manner. ATP hydrolysis then triggers a conformational change to induce protein folding and the release of the substrate. Nucleotide exchange factors (NEFs) facilitate the exchange of ADP for ATP allowing Hsp70 to bind to a new substrate and repeat the cycle.
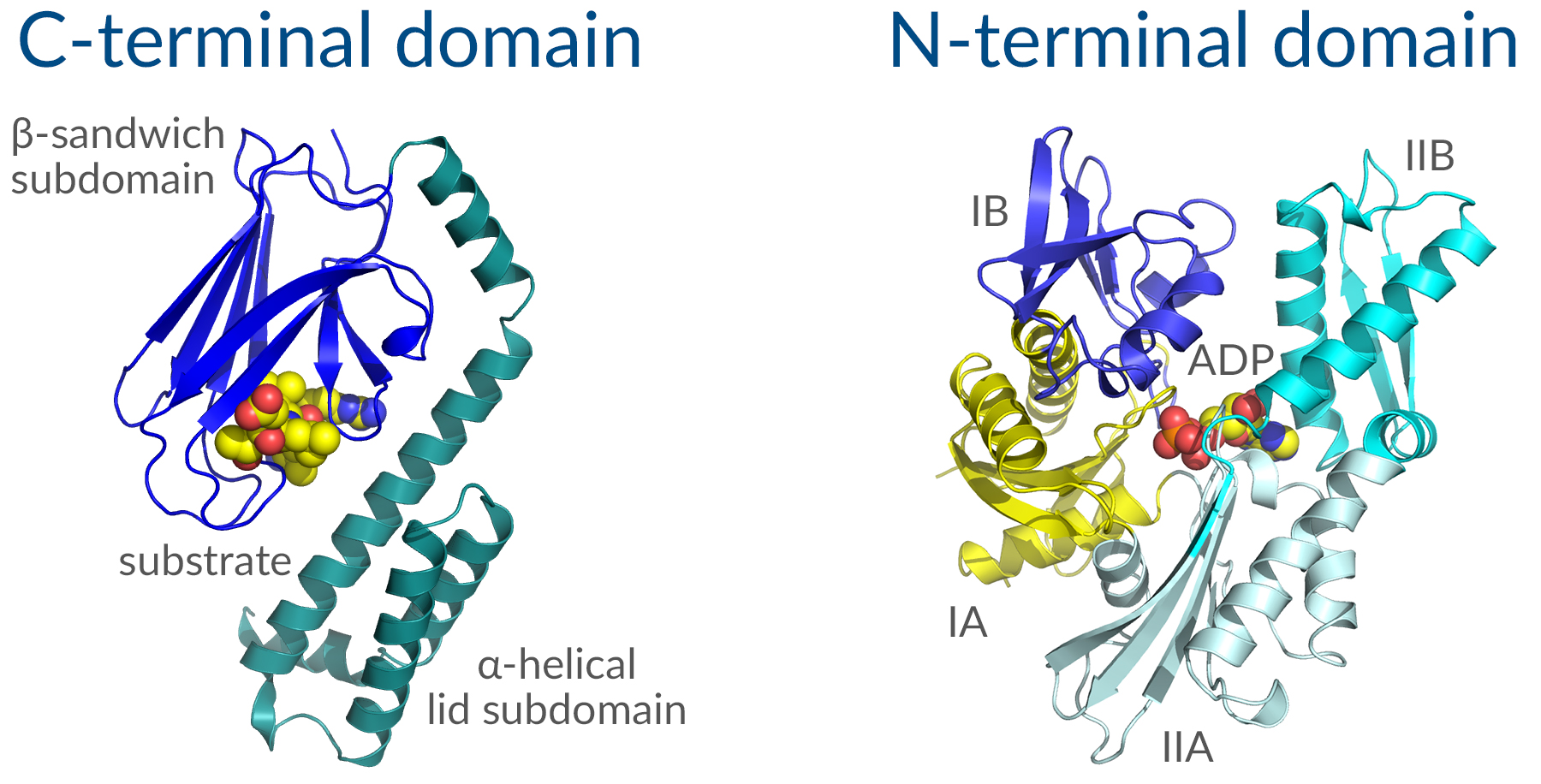
Figure 1. Structure of key Hsp70 domains.
Hsp70 clients include regulators of signal transduction pathways that control cell homeostasis, proliferation, differentiation, and cell death. Together with Hsp90 and other co-chaperones, which will be discussed further below, Hsp70 functions to repress client regulator proteins in an inactive state from which they can be rapidly activated upon appropriate upstream signals when a signal transduction pathway is switched on.1 When misfolded proteins arise from cellular stresses induced by environmental, developmental, or pathological processes, Hsp70 is titrated away from servicing these clients and is thereby disrupted from regulating important signal transduction pathways.
Hsp70 (human recombinant, baculovirus expressed)
Hsc70 (Heat Shock 70 kDa Protein 8, HSPA8, Heat Shock Cognate 71 kDa Protein, or Hsp73)
Constitutively expressed Hsp70 family members are known as heat-shock cognate (Hsc) proteins. Hsc70 is a chaperone from this family that facilitates proper folding of newly translated or misfolded proteins under normal conditions, participating in cellular processes such as endocytosis, nucleocytoplasmic transport, ubiquitylation, and autophagy. It also stabilizes or degrades mutant proteins. Constitutively expressed human Hsc70 has 85% identity with canonical, stress-induced human Hsp70.
Hsp40 and NEFs
The two major classes of co-factors that interact with Hsp70 include J-domain proteins such as Hsp40 and NEFs such as Hsp110. These co-factors enable the specificity and variability of Hsp70 functions. The J-domain module of Hsp40 is a four-helix bundle 70 amino acids in length that contains a tripeptide histidine-proline-aspartic acid (HPD) motif, which interacts with Hsp70 (Figure 2).
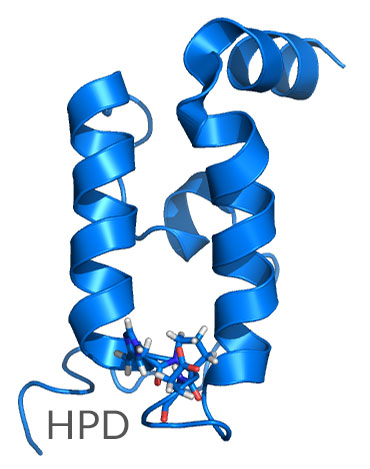
Figure 2. The J-domain of Hsp40 showing the highly conserved HPD motif.
Through this interaction, Hsp40 accelerates the ATPase activity of Hsp70, promoting the conformational change necessary for Hsp70 ATP hydrolysis and the subsequent closure of the Hsp70 α-helical lid domain. NEFs then promote ADP release from the Hsp70 N-terminal ATP-binding domain, resetting the cycle for another round of ATP binding and hydrolysis with a new substrate. Hsp40 also associates with unfolded polypeptide chains and prevents their aggregation.
Hsp90 and co-chaperones
Hsp90
Hsp90 assists with proper protein folding, stabilizes proteins against heat stress, and aids in protein degradation. It also stabilizes many proteins required for tumor growth, which is why Hsp90 inhibitors are explored as anticancer drugs. In mammalian cells, Hsp90AA (inducible) and Hsp90AB (constitutively expressed) genes encode cytosolic Hsp90, yielding Hsp90α and Hsp90β protein isotypes, respectively. These α- and β-forms are thought to be the result of a gene duplication event that occurred early in evolution. Human Hsp90α demonstrates 85% sequence identity to Hsp90β. Both isoforms participate equally in multichaperone complexes, but have noticeably different behaviors with respect to substrate interactions under stress conditions, as significantly more Hsp90α is present after heat shock.2
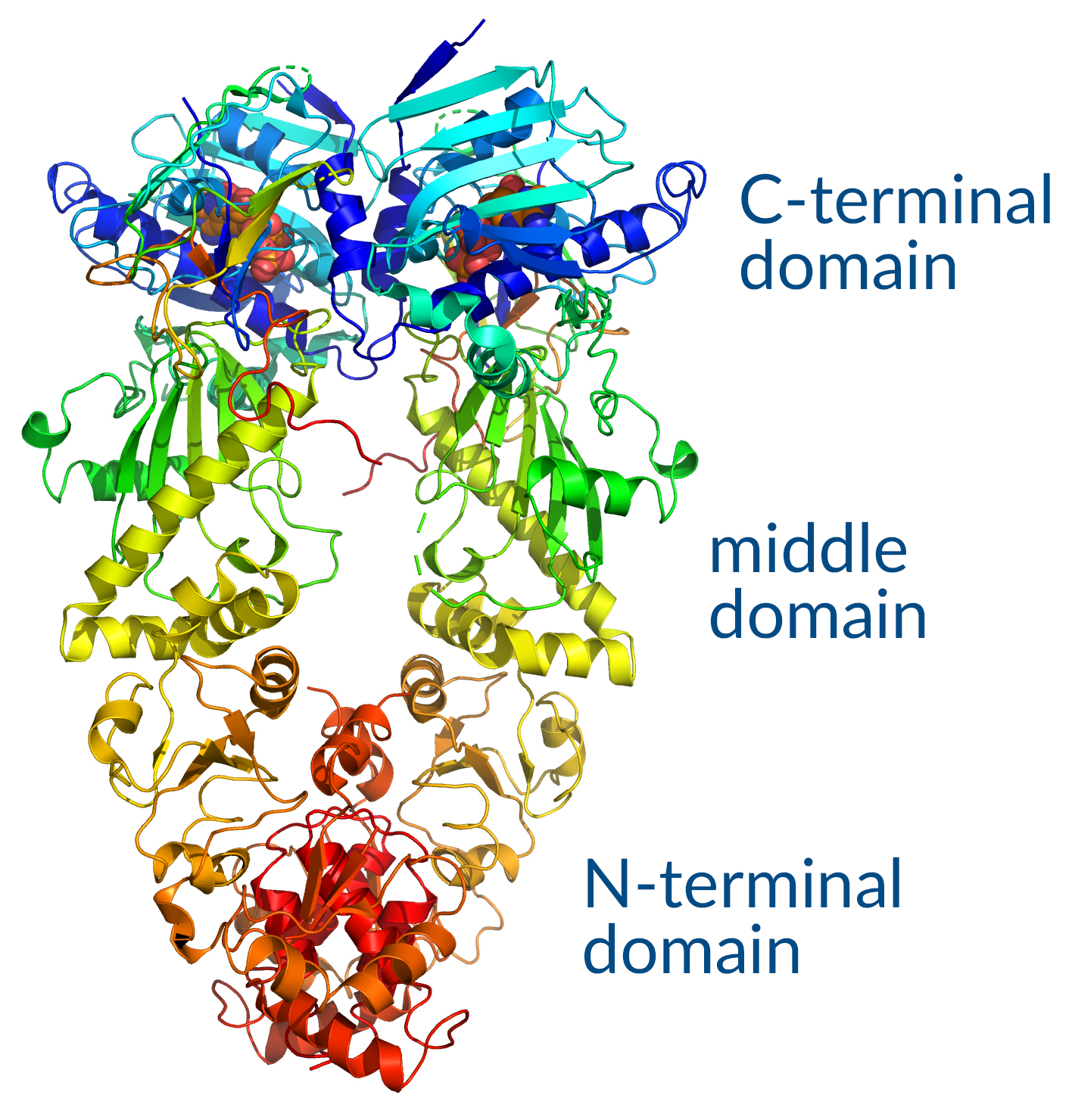
Figure 3. Hsp90 functional domains.
Hsp90 is composed of an N-terminal ATP-binding domain and a C-terminal domain that contains a dimerization region followed by the residues EEVD (Figure 3). Similar to Hsp70, this sequence binds to tetratricopeptide repeat (TPR) domains of co-chaperones. The middle domain connecting N- and C-terminals comprises a charged linker region that is important for flexibility, allowing for conformational changes needed for co-chaperone interaction and ATPase activity. Hsp90 is unique from Hsp70 in that it participates in the final maturation of proteins and the assembly of macromolecular structures, interacting with a more select group of client proteins. These substrates include kinases and transcription factors, though few of these targets have been thoroughly characterized. Hsp90 functions in an ATP-dependent cycle that both controls and is influenced by a complex network of co-chaperones, which promote transition between functional Hsp90 states required for substrate maturation. Hsp90 is also regulated post-translationally through phosphorylation, acetylation, and S-nitrosylation.
HOP (Hsp70/Hsp90 Organizing Protein, Stress-Induced Phosphoprotein 1, STI1, or STIP1)
HOP acts as a co-chaperone forming a reversible complex with Hsp70 and Hsp90 to assist in handing off proteins from Hsp70 to Hsp90 (Figure 4). It bears three TPR domains (TPR1, TPR2a, and TPR2b), which act as distinct substrate binding regions. Prior to Hsp90 interacting with its client proteins, Hsp70, along with its co-factors, associate with most of these clients to assemble a pre-folding complex. Once assembled, the client-bearing Hsp70 is brought into proximity of Hsp90 dimers through the actions of HOP, where the client is released to Hsp90.
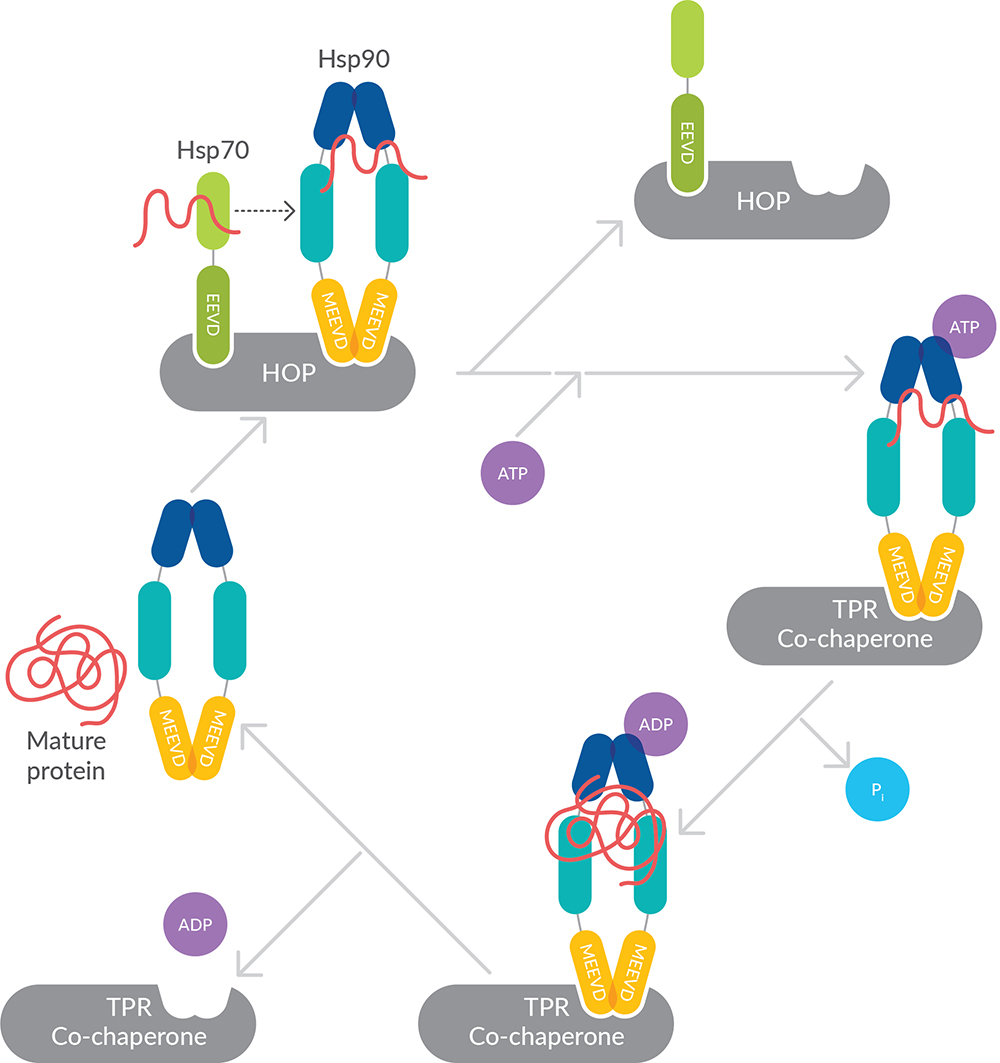
Figure 4. Cooperation of Hsp70, Hsp90, and HOP to mediate protein folding.
HOP functions in two capacities. First, it is an early acting co-chaperone that inhibits ATPase activity, binding Hsp90 in an open conformation and promoting asymmetric assembly of Hsp90 with its co-chaperones. Inhibition of the Hsp90 ATPase is important for client transfer. Subsequently, HOP facilitates the transfer of substrates from Hsp70 to Hsp90 through its ability to simultaneously bind Hsp90 and Hsp70 at distinct tetratricopeptide repeats. TPR1 and TPR2b bind Hsp70, while TPR2a binds Hsp90.
Small Hsps
Small Hsps are important for promoting protein solubility when heat or other stressors lead to cytosolic protein unfolding. They function independent of ATP, uniting into large and dynamic oligomers and binding to unfolded proteins promiscuously to prevent their aggregation and keep them in a folding-competent state.3 They protect cells from protein losses or toxicity caused by aggregation, but must eventually release their aggregation-prone clients to other downstream chaperones that facilitate folding. Small Hsps are thought to enable stress resistance, inducing thermotolerance and inhibiting apoptosis.
Hsp27 (Heat Shock Protein Beta-1, HSPB1, Hsp25, or Hsp28)
Hsp27 is the most ubiquitous of the small Hsps and plays a role in stress tolerance and actin organization. In lieu of ATP hydrolysis, its chaperone activity is regulated by phosphorylation. In response to environmental stress, phosphorylated Hsp27 translocates from the cytoplasm to the nucleus, functioning as a molecular chaperone that promotes proper protein folding. It plays an important role in the differentiation of a wide variety of cell types but can also promote cancer cell proliferation and metastasis, protecting cancer cells from apoptosis. Mutations in the gene encoding Hsp27 are associated with Charcot-Marie-Tooth disease and distal hereditary motor neuropathy, both of which are caused by defects in neuronal proteins.
Chaperonins
Chaperonins offer protection (e.g., prevent aggregation and protein misfolding) by providing favorable conditions for folding unfolded or partially unfolded proteins. They accomplish this by forming a double-ring structure that resembles two donuts stacked on each other (Figure 5). A lid structure covers the central cavity of these rings, creating a complex capable of enclosing protein substrates. Proteins are folded within the central cavity of this complex in an ATP-dependent manner and then released back into the cytosol. Interaction with additional chaperones confers specificity for those substrates to be folded. There are two main classes of chaperonins: group I (homomultimers found in bacteria and organelles such as chloroplasts and mitochondria) and group II (hetero-oligomers found in the cytosol of eukaryotes and archaea). The best characterized of these is the group I mitochondrial chaperonin Hsp60 and its co-chaperonin Hsp10, which are described further below.
Organellar Chaperones
Because proteins in the endoplasmic reticulum (ER) and mitochondria are physically separated from the cytosolic protein quality control machinery, a dedicated set of molecular chaperones assist in the import of proteins and unique folding environment of these organelles. Both the ER and mitochondria use the proteasome to degrade damaged proteins, so chaperones must also engage in export of these items back to the cytosol. The expression of organelle chaperones is largely regulated by the unfolded protein response in the ER and the retrograde response in the mitochondria.
GRP78 (Binding Immunoglobulin Protein, BiP, 78 kDa Glucose-Regulated Protein, Heat Shock 70 kDa Protein 5, or HspA5)
GRP78 is a member of the Hsp70 family of molecular chaperones that is in the ER lumen. It binds newly synthesized proteins as they are translocated into the ER and maintains them in a state competent for subsequent folding and oligomerization. GRP78 is also an essential component of the translocation machinery, playing a role in retrograde transport of aberrant proteins destined for proteasomal degradation. While GRP78 is expressed under all growth conditions, its synthesis is markedly induced under conditions that lead to the accumulation of unfolded polypeptides in the ER.
Hsp60 (60 kDa Chaperonin, Chaperonin 60, Cpn60, HuCHA60, Mitochondrial Matrix Protein P1, P60 Lymphocyte Protein, or HSPD1)
Hsp60 belongs to the chaperonin family and functions in mitochondrial protein import and macromolecular assembly. It can facilitate the correct folding of imported proteins and prevent misfolding or promote the refolding and proper assembly of unfolded polypeptides generated in the mitochondrial matrix under stressful conditions. The functional complex of these chaperonins consists of two stacked heptameric rings that act in conjunction with co-chaperonin Hsp10.
Hsp10 (HSPE1, 10 kDa Chaperonin, Chaperonin 10, CPN10, Early Pregnancy Factor, or EPF)
Hsp10 forms a lid for the donut-shaped Hsp60 structure to facilitate an ideal environment for folding imported proteins. In a recurrent cycle, the Hsp60 double-ring complex binds an unfolded protein, followed by the binding of ATP and association with two heptameric rings of Hsp10 (Figure 5). This leads to sequestration of the substrate protein in the inner cavity of Hsp60, creating an environment where the protein can fold undisturbed by other cell components. As ATP is hydrolyzed in the Hsp60 subunits, the chaperonin rings dissociate, releasing ADP as well as the folded substrate protein and freeing the Hsp60 to start the sequence anew.
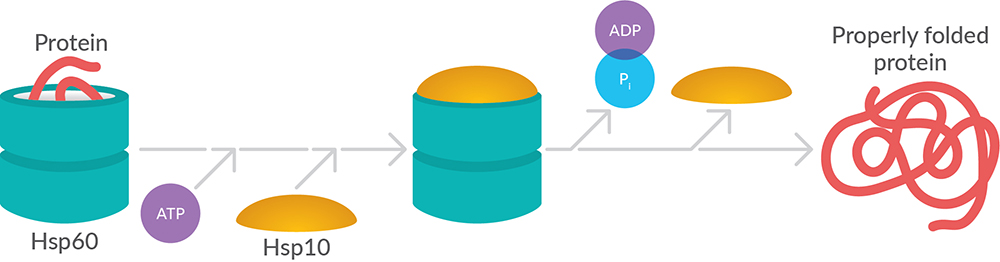
Figure 5. Chaperonins form hollow structures enabling proteins to be folded in a permissive environment.
Outside of the mitochondria and its co-chaperonin responsibility, extracellular Hsp10 possesses immunomodulatory properties, participates in proliferation/differentiation mechanisms of both normal and tumor cells, and is identical to the protein previously identified as early pregnancy factor that appears as a growth factor in maternal serum 24 hours after fertilization.4
Miscellaneous Chaperones
HO-1 (Heme Oxygenase-1, HMOX1, or Hsp32)
HO-1 is induced by reactive oxygen species or hypoxia and degrades pro-oxidant heme, leading to the formation of the antioxidant bilirubin. HO-1 was first observed in cells exposed to heavy metals as an Hsp32 cognate exquisitely sensitive to oxidative stress but is now characterized as a microsomal enzyme that is central to cellular defense mechanisms. Whereas HO-1 is stress-responsive, a second form, HO-2, catalyzes the same reaction but functions constitutively, maintaining cellular heme homeostasis and inactivating free radicals under normal conditions to confer protection against oxidative stress.5
Transcriptional Regulation
The increased expression of Hsps in a stressed cell is mediated primarily by heat shock transcription factors (Hsfs). Among the four members of the Hsf family in vertebrates and plants, Hsf1 bears the primary role in transcriptional regulation of Hsp expression. During stress, accumulation of misfolded proteins rapidly activates Hsf1. Under non-stress conditions, the heat shock response is self-regulated through repression. Hsp70, in conjunction with the Hsp90 chaperone complex, serves as a trans-acting Hsf1 repressor, preventing the transcriptional activation of Hsf1 through a mechanism involving their ATPase activity. As such, Hsf1 can be activated by certain Hsp90 inhibitors that target the N-terminal ATPase (e.g., geldanamycin and radicicol), which poses a problem for the effectiveness of these agents in certain disease treatments due to the ultimate initiation of a protective response.6 During a heat shock response, the accumulation of unfolded or damaged proteins works to titrate the chaperone machinery away from Hsf-1, allowing derepression of the transcription factor.
Hsf1 Monoclonal Antibody (Clone 10H8)
Hsf2 Monoclonal Antibody (Clone 3E2)
View a complete list of Cayman’s Heat Shock Protein research tools.
Download the Heat Shock Protein Research Tools Brochure (PDF).
References
1. Mayer, M.P. and Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell Mol. Life Sci. 62(6), 670–684 (2005).
2. Taherian, A., Krone, P.H., and Ovsenek, N. A comparison of Hsp90α and Hsp90β interactions with cochaperones and substrates. Biochem. Cell Biol.86(1), 37-45 (2008).
3. Mymrikov, E.V., Daake, M., Richter, B., et al. The chaperone activity and substrate spectrum of human small heat shock proteins. J. Biol. Chem.292(2), 672-684 (2017).
4. Corrao, S., Campanella, C., Anzalone, R. et al. Human Hsp10 and early pregnancy factor (EPF) and their relationship and involvement in cancer and immunity: Current knowledge and perspectives. Life Sci.86(5-6), 145-152 (2010).
5. Maines, M.D. and Panahian, N. The heme oxygenase system and cellular defense mechanisms. Do HO-1 and HO-2 have different functions? Adv. Exp. Med. Biol.502, 249–272 (2001).
6. McConnell, J., Wang, Y., and McAlpine, S. Targeting the C-terminus of Hsp90 as a cancer therapy. In Heat Shock Protein Inhibitors. Success Stories. McAlpine S.R. and Edkins A.L. (eds.), 19, 1-20, Springer (2016).

